The Role of TYK2 in Immunology
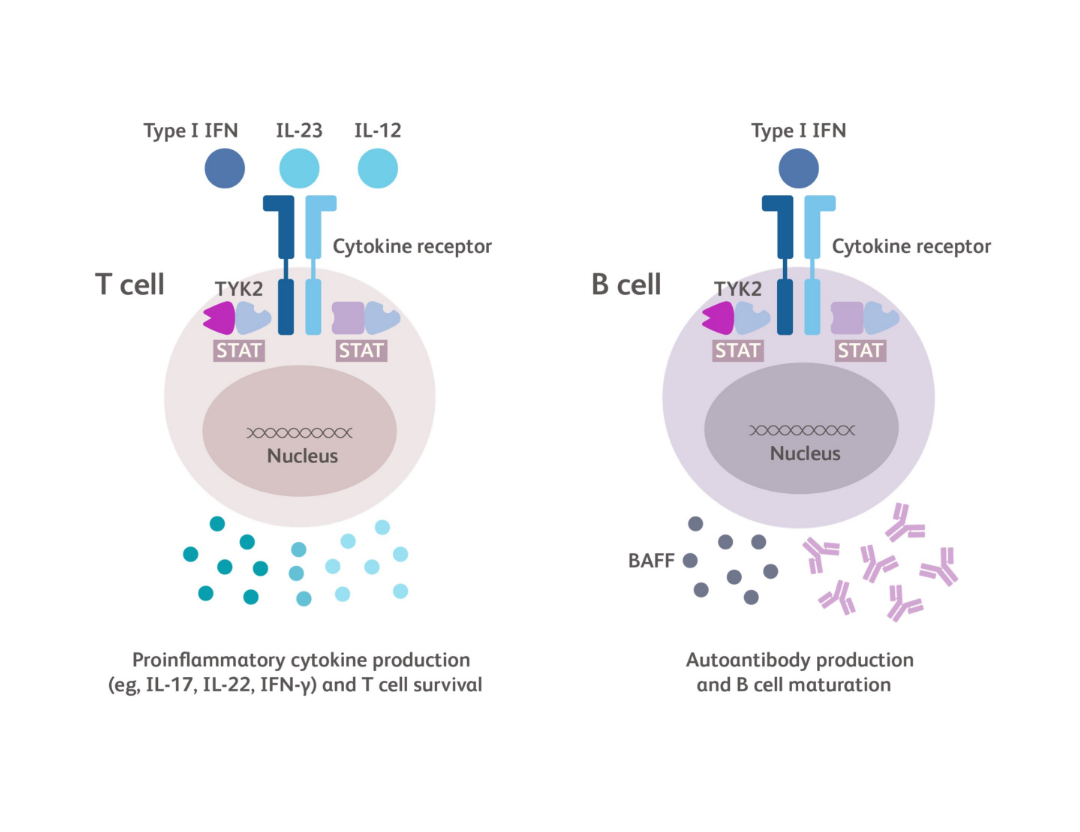
TYK2 is an intracellular kinase in the Janus kinase (JAK) family of enzymes. TYK2 and JAK1/2/3 proteins work in pairs (dimers) to relay immune signals initiated by specific cytokines.1
TYK2 is a central link between certain cytokines, such as IL-23, IL-12 and Type I IFN, and the downstream effects of these cytokines.2
TYK2 and TYK2-dependent cytokines may play a key role in the pathophysiology of various rheumatic diseases such as psoriatic arthritis, systemic lupus erythematosus, Sjögren’s disease, dermatomyositis, and systemic sclerosis.3-7
Impact of TYK2 gene variations
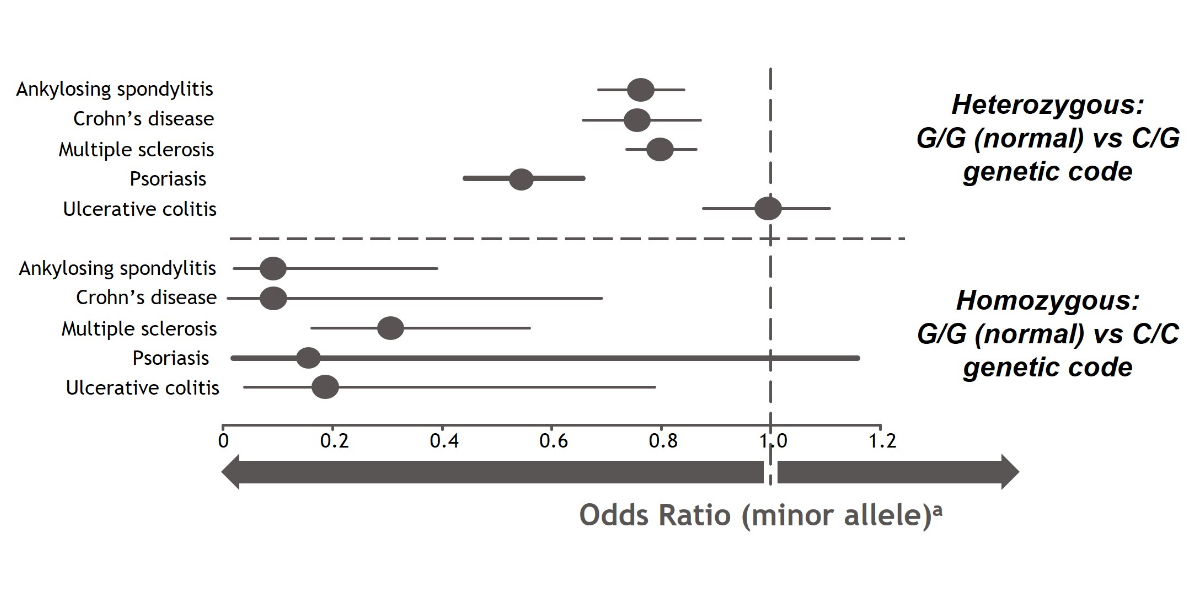
A genome-wide association study (GWAS) from Dendrou, et al used data from the UK BioBank to illustrate the impact of TYK2 gene variations on the risk of developing multiple autoimmune diseases. These genetic variations have been suggested to reduce the function of TYK2 in mediating cytokine signaling, potentially altering the associated downstream consequences.2
TYK2 loss-of-function mutations at a location called rs34536443 were associated with a reduced risk of developing certain inflammatory and autoimmune diseases. When both copies of the TYK2 gene had this loss-of-function mutation (homozygous), the risk for developing these inflammatory and autoimmune diseases was even further reduced. Additional research is needed to better understand the role of TYK2 in these diseases.2
Associations of a TYK2 gene variant with the risk of developing selected inflammatory and autoimmune disorders2,a


aOdds ratio is a measure of the association between an exposure and an outcome. The odds ratio represents the likelihood that an outcome (eg, disease) will occur given a particular exposure (ie, genetic variation), compared with the odds of the outcome occurring in the absence of that exposure. An odds ratio of < 1.0 suggests that an outcome is less likely while an odds ratio of > 1.0 suggests that an outcome is more likely.12
These links are meant for informational purposes only. Bristol Myers Squibb does not endorse the content contained within these links.
Ghoreschi K, et al. Immunol Rev 2009;228(1):273-87.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2782696/Hammarén HM, et al. Cytokine 2019;118:48–63.
https://www.sciencedirect.com/science/article/pii/S1043466618301273?via%3DihubDendrou CA, et al. Sci Transl Med 2016;8:363ra149.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5737835/Morris R, et al. Protein Sci 2018;27:1984–2009.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6237706/TYK2 and JAK1/2/3
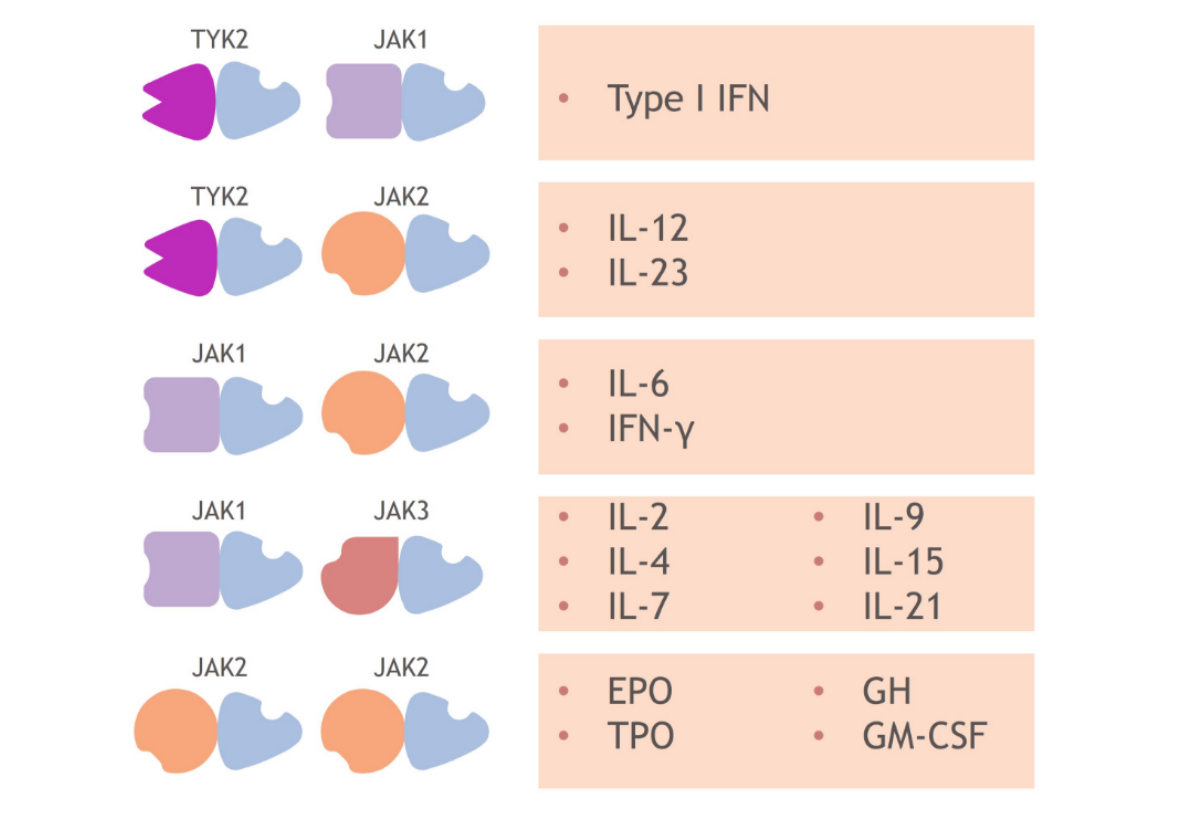
Each member of the TYK2/JAK family pairs with another member to relay signals initiated by different cytokines.1,13-16 This chart shows examples of which cytokines are associated with which kinase pairs.1,8
For example, pairs containing TYK2 mediate the signaling of cytokines such as Type I IFN, IL-23, and IL-12,1 while pairs containing 2 JAK2 members mediate signaling from erythropoietin, growth hormone, thrombopoietin, and granulocyte-monocyte colony-stimulating factor.1,8
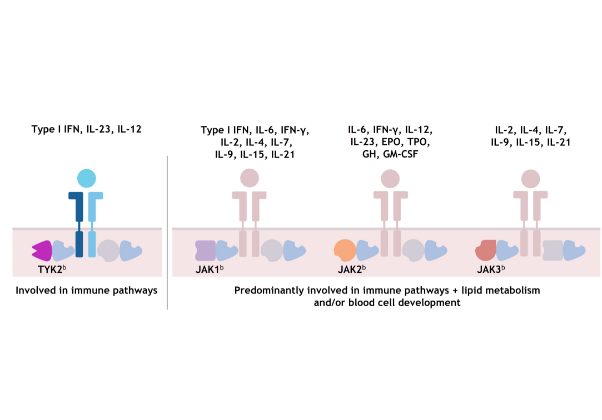
As a result, each member of the TYK2/JAK family is active in mediating cytokines involved in different immune system functions; TYK2 has not been shown to be required for pathways outside of the immune system.8,17,18
JAK1, JAK2, and JAK3 combinations each mediate cytokines involved in additional functions, including lipid metabolism and blood cell development.1,8,14,1719,21-23
aPlease note that this list of cytokines and effects modulated by different TYK2/JAK and JAK/JAK pairs is not exhaustive. Certain cytokines might also be modulated by JAK and TYK2 trimers.8
bJAK family dimer pairs are cytokine specific and include TYK2/JAK1, TYK2/JAK2, JAK1/JAK3, and JAK2/JAK3.
TYK2 and JAK 1/2/3 signaling1,8,14,17,20–22,a
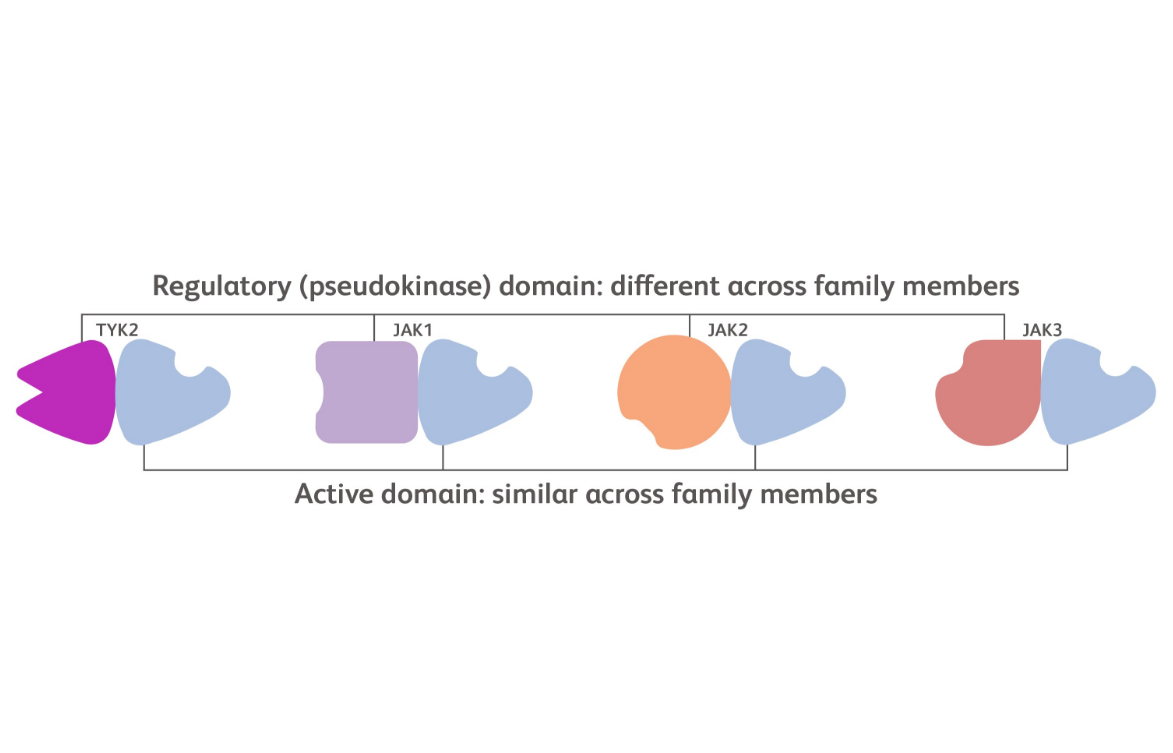
Each kinase in the JAK family includes 2 domains: an active domain where ATP binding occurs and a regulatory domain that plays a supportive function.15
The active domain structure is similar across each of the JAK family members while the regulatory domain is structurally more distinct.15
These links are meant for informational purposes only. Bristol Myers Squibb does not endorse the content contained within these links.
Ghoreschi K, et al. Immunol Rev 2009;228(1):273-87.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2782696/Hammarén HM, et al. Cytokine 2019;118:48–63.
https://www.sciencedirect.com/science/article/pii/S1043466618301273?via%3DihubMorris R, et al. Protein Sci 2018;27:1984–2009.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6237706/Diseases of Interest
- TYK2 in PsA
- TYK2 in SLE
- TYK2 in SjD
The Role of TYK2 in PsA
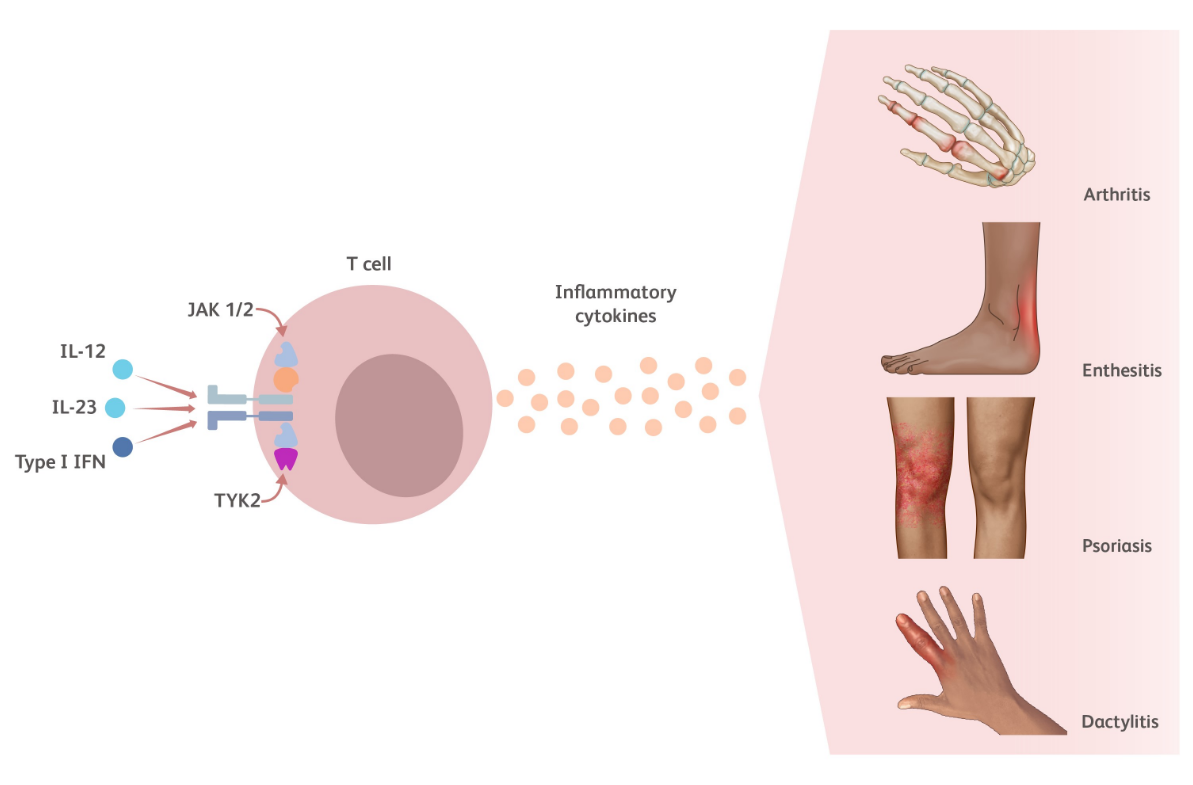
TYK2 is an intracellular kinase critical for the relay of immune signals initiated by proinflammatory cytokines such as IL-23, IL-12, and the Type I IFNs. Additional TYK2 pathway cytokines relevant to psoriatic arthritis (PsA) include IL-22 and IFN-λ.23-25
- IL-23 induces the production of both IL-17 and IL-2226
- IL-12 drives the production of IFN-γ27
- Type I IFNs play a role encouraging the survival, maturation, and activation of different cell types27,28
Cytokines modulated by the TYK2 pathway play a key role in PsA pathophysiology including but not limited to psoriasis, arthritis, enthesitis, dactylitis, fatigue, and pain.11,14,29-31
TYK2 in the genetics of patients with PsA
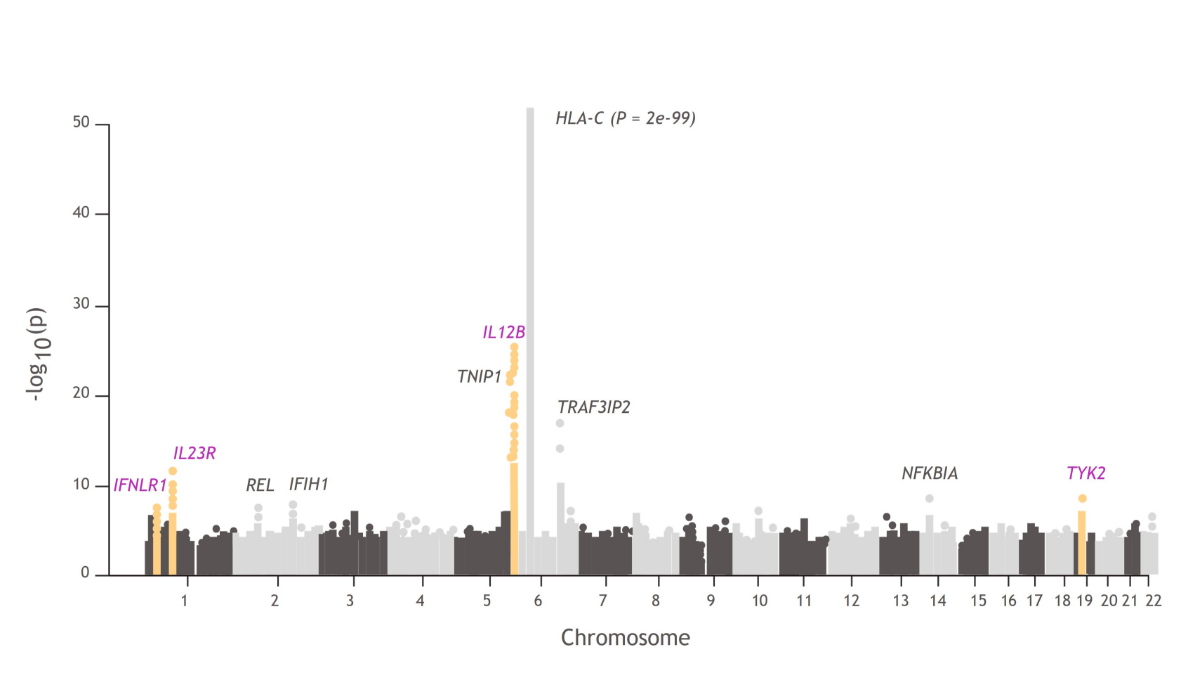
In a PsA GWAS, 4 of 10 regions were associated at genome-wide significance with the TYK2 pathway:
- TYK2, IL23R, IL12B, and IFNLR13,32,33
Manhattan plots like this one illustrate the findings from GWAS, which are used to understand the cellular and molecular pathways associated with disease pathophysiology compared with healthy controls.34
These links are meant for informational purposes only. Bristol Myers Squibb does not endorse the content contained within these links.
Schinocca C, et al. Front Immunol 2021;12:637829.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7937623/Stuart PE, et al. Am J Hum Genet 2015;97:816–836.
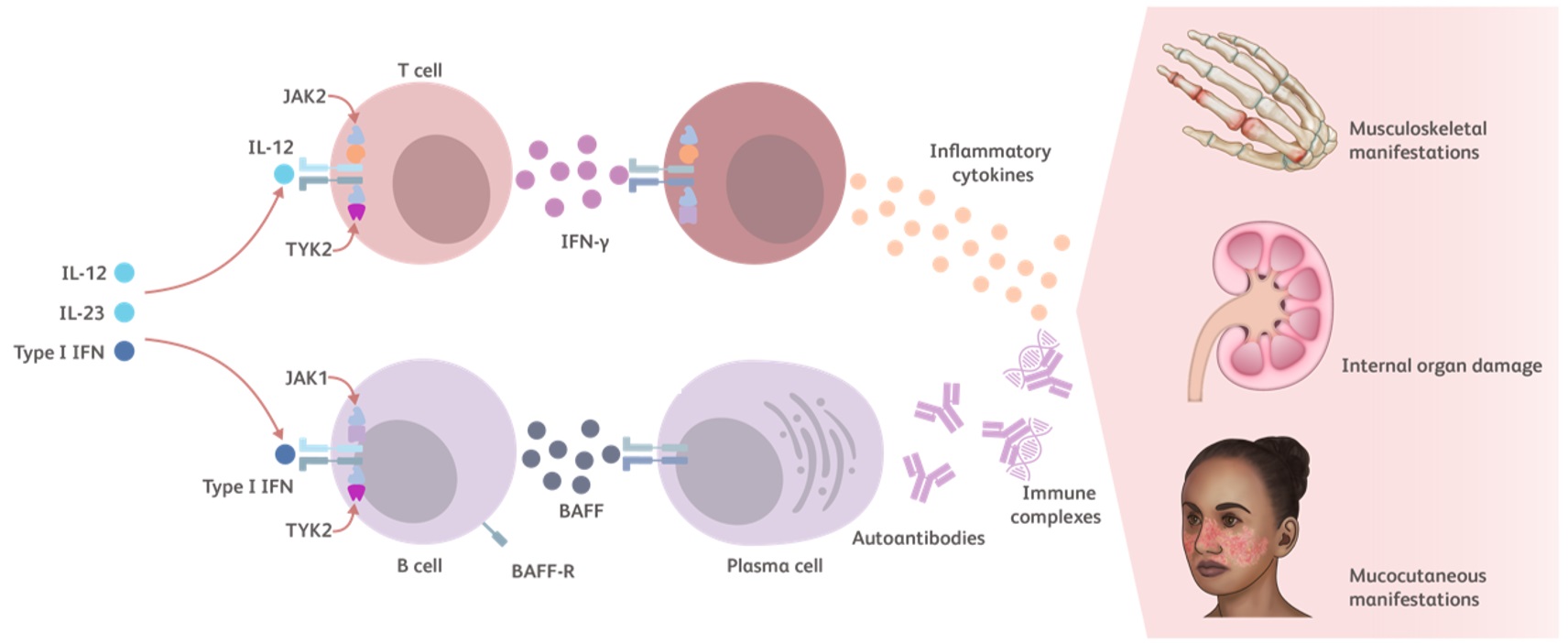
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4678416/The Role of TYK2 in SLE
TYK2 is an essential kinase for intracellular immune signaling initiated by proinflammatory cytokines such as Type I IFNs, IL-23, and IL-12. Additional TYK2 pathway cytokines include IL-10 and IFN-λ.23-25
- Type I IFNs have several pathophysiological and proinflammatory mechanisms in lupus27,36,37:
- Increase the survival of various cell types
- Promote B-cell survival and lead to development of plasma cells and autoantibody production
- IL-12 induces the production of IFN-γ27,38,39
- IL-10 induces the production of autoantibodies by plasma cells27,40
- Together, Type I IFN and BAFF promote the differentiation of B cells into plasma cells, production of autoantibody, and immune complex deposition1,41
Cytokines modulated by the TYK2 pathway contribute to the inflammatory cycle of lupus that leads to autoimmunity, chronic tissue injury (systemic disease) and damage to the skin, kidneys, and/or joints (local manifestations).27,42
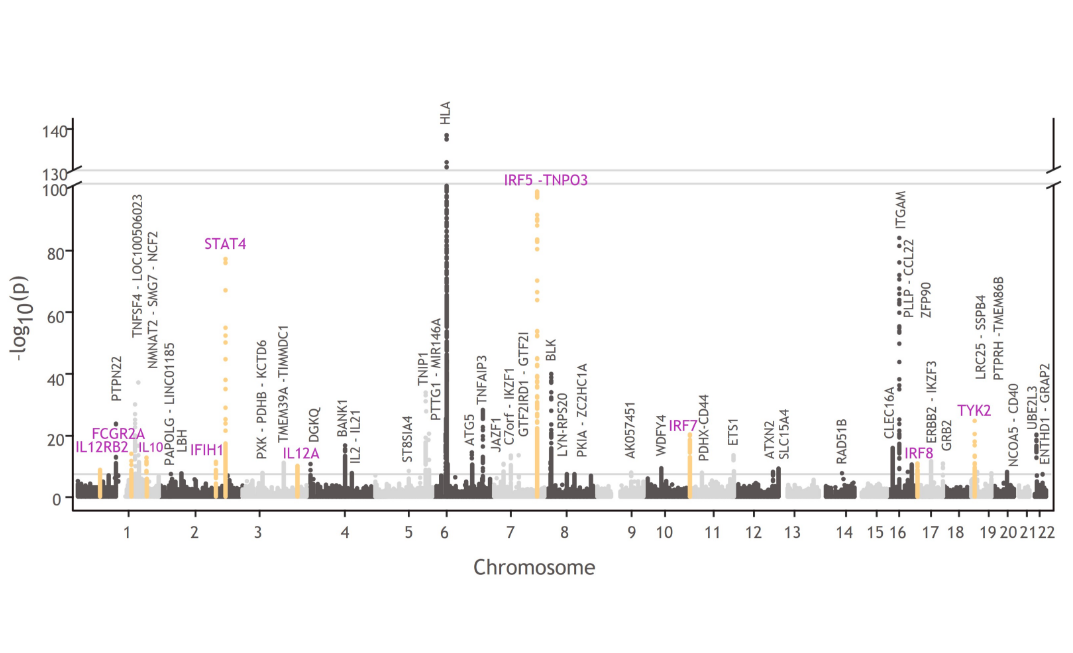
TYK2 in the genetics of patients with SLE
TYK2 and other genes involved in TYK2-mediated cytokine signaling are associated with lupus pathophysiology:1,4,27,43-47
- TYK2, STAT4, IRF5, IRF7, IRF8, IFIH1, IL12A, IL12RB2, FCGR2A, and IL10
Manhattan plots like this one illustrate the findings from GWAS, which are used to understand the cellular and molecular pathways associated with disease pathophysiology compared with healthy controls.34
These links are meant for informational purposes only. Bristol Myers Squibb does not endorse the content contained within these links.
Langefeld CD, et al. Nat Commun 2017;8:16021.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5520018/Rönnblom L, Leonard D. Lupus Sci Med 2019;6(1):e000270.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6703304/Aringer M, Johnson SR. Rheumatology (Oxford) 2020;59(Suppl5):v4-v11.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7719035/Oke V, et al. Arthritis Res Ther 2019;21(1):107.
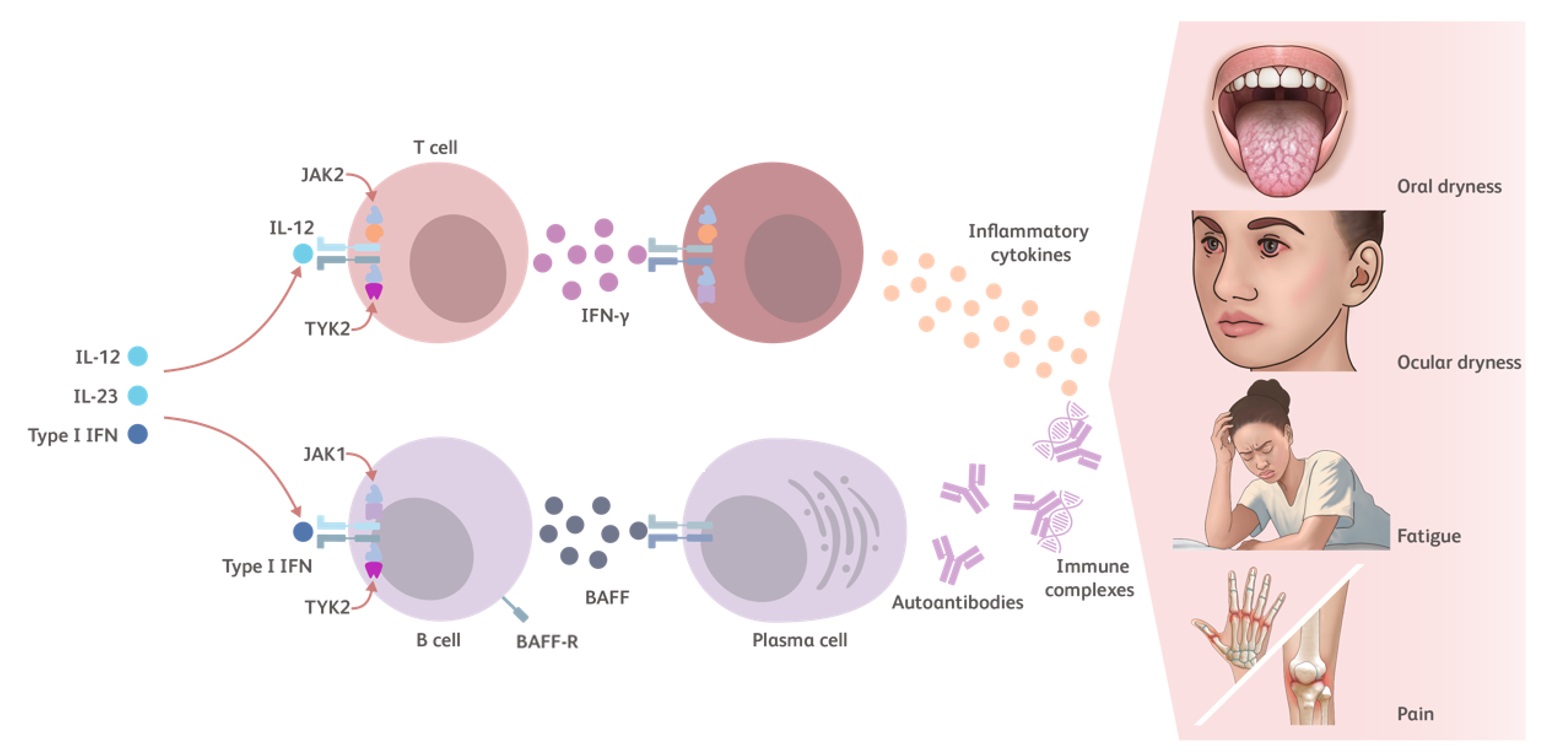
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6489203/The Role of TYK2 in Sjögren’s Disease
TYK2 is an intracellular kinase that relays immune signals initiated by proinflammatory cytokines such as Type I IFNs, IL-23, and IL-12. Additional TYK2 pathway cytokines include IL-10 and IFN-λ.23-25
- Type 1 IFNs have several pathophysiological and proinflammatory mechanisms in Sjögren’s disease57
- Increase the survival of various cell types
- Promote B-cell mediated autoantibody production
- IL-12 induces IFN-γ production58
- IL-10 contributes to the production of autoantibodies by plasma cells27
- IL-10 levels are increased in patients with Sjögren’s disease and positively correlate with anti-Ro and anti-La antibody levels59
- Together, Type I IFN and BAFF promote the differentiation of B cells into plasma cells, production of autoantibody, and immune complex deposition1,41
Cytokines modulated by the TYK2 pathway have been shown to play a key role in Sjögren’s disease pathophysiology, including but not limited to the development of dryness and fatigue.23,24,60-65
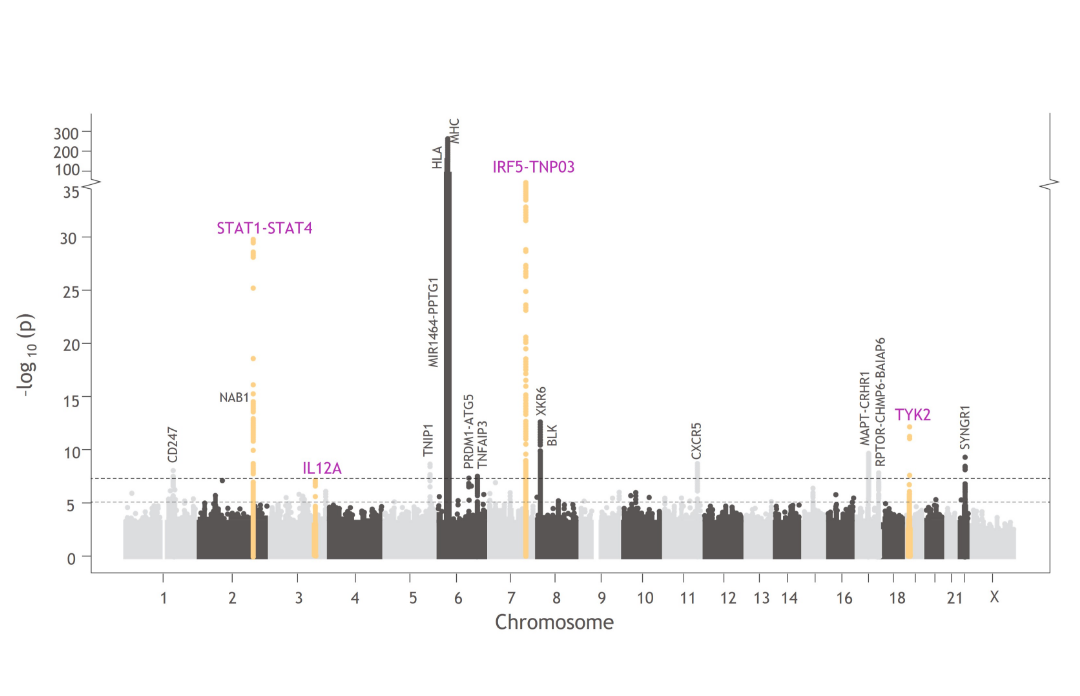
TYK2 in the Genetics of Sjögren’s Disease
TYK2 and other genes involved in TYK2-mediated cytokine signaling are associated with Sjögren’s disease pathophysiology, including TYK2, IRF5, IL12A, and STAT1-STAT4.1,5,44,66,67
Sjögren’s Disease and Lupus
The pathophysiology of Sjögren’s disease largely overlaps with lupus pathophysiology; they share clinical features and may coexist.68
Manhattan plots like this one illustrate the findings from GWAS, which are used to understand the cellular and molecular pathways associated with disease pathophysiology compared with healthy controls.^34
These links are meant for informational purposes only. Bristol Myers Squibb does not endorse the content contained within these links.
Khatri B, et al. Nat Commun 2022;13:4287.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9329286/Trutschel D, et al. Arthritis Rheumatol 2022;74(12):1991-2002.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10092541/